A Trump-appointed federal judge is expected to hand down a decision this month that could block access to mifepristone, one of the two drugs used in the recommended medication abortion regimen. The consequences of such a decision would not only be seen in states with abortion restrictions but in states where abortions still remain legally accessible, too.
The Food and Drug Administration (FDA) approved mifepristone for the medical termination of pregnancy over two decades ago. But a lawsuit filed last November alleges that the longstanding approval should be revoked because it was allegedly based on incomplete data. The anti-abortion organization Alliance for Hippocratic Medicine claims that the FDA failed to protect women when it approved the drug.
"The lawsuit alleges that the side effects of mifepristone were not reported adequately 23 years ago when they were reported to the FDA, or that there are more side effects," Seema Mohapatra, a professor of law at SMU Dedman School of Law, told Salon. "That is actually scientifically disputed, there are tons of studies showing how safe and effective mifepristone is."
Indeed, last week, the American College of Obstetricians and Gynecologists, the American Medical Association and the Society for Maternal-Fetal Medicine, and nine other prominent medical organizations submitted an amicus brief including a list of evidence that shows mifepristone is safe and effective.
"Medication abortion including mifepristone is safe and effective," the brief states. "This is not an opinion—it is a fact based on hundreds of medical studies and vast amounts of data amassed over the course of two decades; the FDA based its initial approval on robust evidence which showed mifepristone was extremely safe."
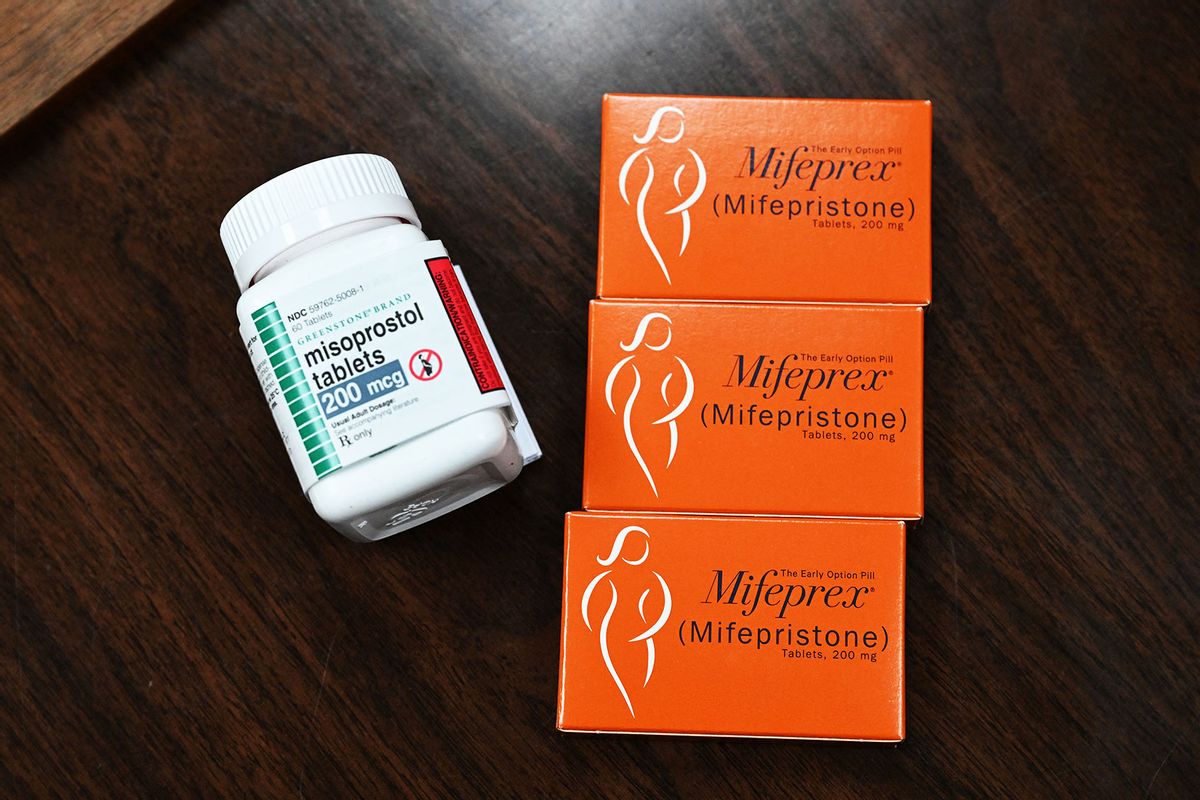
Medication abortions occur through the brand name drug Mifeprex. In the two-step process, a pregnant person first takes a mifepristone pill, which is the drug at the center of the lawsuit. Either 24 to 48 hours later, a second pill containing misoprostol is taken. Medication abortion works up to 70 days after the first day of a person's last period — usually when they are 10 weeks pregnant. According to data from the Centers for Disease Control and Prevention, medication abortions account for an estimated 42 percent of all abortions in the United States.
Without the mifepristone pill, the first step in the process, the only drug that will be available for medication abortions is misoprostol. Will this still work?
The short answer is yes.
A misoprostol-only medication abortion, it should be noted, is not FDA-approved, so technically this use is off-label. While mifepristone blocks progesterone, a hormone needed to support pregnancy, misoprostol contracts and dilates the cervix to expel the embryo. Many studies have shown that misoprostol is safe and effective at terminating pregnancy in the first trimester alone. However, some studies have found that the regimen in taking both mifepristone and misoprostol is more effective than taking misoprostol alone. On Feb. 6, University of Texas-Austin researchers published a peer-reviewed study on the use of misoprostol alone for abortion and concluded it was 88% effective. The research was based on data from the telemedicine abortion provider Aid Access. Misoprostol is most commonly sold under the brand name Cytotec.
"Self-managed medication abortion using misoprostol provided by an online telemedicine service has a high rate of effectiveness and a low rate of serious adverse events," the authors concluded. "As mifepristone continues to be over-regulated and the 2022 US Supreme Court ruling allows states to severely restrict access to in-clinic abortion care, this regimen is a promising option for self-managed abortion in the US."
Misoprostol is a widely available medication, and its primary use is to treat gastric ulcers. Women in Brazil in the 1980s saw an opportunity with its warning label, which stated that the drug could cause a miscarriage.
"We've seen in other countries that have had similar ideological issues, like Brazil, where we've seen miso-only abortions be effective," Mohapatra said. "So it was actually developed for gastric ulcers, and then it was an off-label use in terms of using it for abortion."
Mohapatra emphasized this is very common.
"Off-label use of FDA-approved drugs is very common, a lot of times it's the most common way a drug is used," Mohapatra said. "We've seen this in other contexts."
Pro-abortion advocates anticipate it will be easier for clinics in states where abortion care is still accessible to pivot to misoprostol-only abortions instead of in-clinic procedural ones.
"It would be difficult, if not outright impossible, for providers that only offer medication abortion using mifepristone to switch to offering procedural abortions instead," Guttmacher Institute said in an updated analysis. "Some of these providers will pivot to offering medication abortion using only misoprostol, while others will be forced to stop offering abortion services entirely."
Still, a misoprostol-only option isn't ideal.
"While 98% of medication abortions in the United States in 2020 used a regimen of mifepristone and misoprostol in combination, misoprostol can be used on its own to end a pregnancy," Guttmacher Institute stated. "If mifepristone becomes unavailable, it is unclear whether all current providers using the two-drug regimen would offer abortion care using only misoprostol and to what extent patients would take up this method."
Read more
about reproductive rights


Shares