The intestinal microbiota is the set of bacteria and viruses that live inside your gut. Microbiota perform a variety of functions, including digesting food and protecting against specific pathogens.
There are several things that can disrupt the gut microbiota, including diet, alcohol consumption, antibiotics and inflammatory bowel disease. These cause imbalances, known as "dysbiosis" which, in turn, are associated with a wide array of chronic diseases.
In the last decade, the results of hundreds of studies in animal models have suggested that gut dysbiosis may play a role in several metabolic disturbances. Furthermore, in rodents, the implantation of certain bacteria could influence weight and metabolic profile. What's more, transferring the gut microbiota from a thin mouse to a heavy mouse allows it to lose weight. Is this too good to be true?
Respectively a student and a full professor in the Department of Medicine at Université Laval, our goal is to identify new therapeutic targets for chronic diseases and healthy life expectancy by using an approach based on genetic epidemiology. This short article aims to summarize and contextualize our recent research work on the gut microbiota.
The importance of a causal link
Scientists have suggested that eating certain foods such as dietary fiber, antioxidant-rich fruits and red meat may have an effect on the gut microbiota. Some even suggest that microbiota could become a therapeutic target for the prevention or treatment of certain chronic diseases.
For the microbiota to become a therapeutic target of interest, it is essential to establish a causal link between the characteristics of the gut microbiota and chronic diseases. A causal link suggests that modifying the microbiota would decrease the risk of developing a disease. However, while several observational (non-experimental) studies in humans have identified statistical associations between various markers of gut microbiota and chronic disease, causality has not been clearly established.
For example, it is not known whether gut dysbiosis is the cause or consequence of disease (reverse causation). It is also not known whether both are influenced by other "confounding" factors that are associated with both gut microbiota and chronic disease. One could think, for example, of the quality of our diet, our weight or our alcohol consumption.
So, the aim of our work was to determine whether there is a direct and causal relationship between gut microbiota and metabolic markers such as weight, eight chronic diseases and human longevity using a genetic approach called Mendelian randomization.
The power of genetic data
Mendelian randomization attempts to establish causal links from genetic data. To do this, Mendelian randomization uses genetic variants (frequent changes in our genome sequence called nucleotide polymorphisms) that are strongly associated with a risk factor (gut microbiota), to establish a causal link with a dependent variable (health markers and diseases), as described in a recent article. Since the variations in our genome are established at the time of embryo formation and remain stable throughout our lives, this natural randomization experiment is not subject to reverse causality bias, since the presence of disease does not influence our genetic code. It is also not subject to the effect of confounding factors, since the genetic variations used are specifically associated with the characteristics of the gut microbiota.
We included genetic data from tens of thousands of individuals from several cohorts. We identified genetic variants associated with 10 fecal and blood metabolites. The metabolites included are small molecules produced by the gut microbiota that have previously been associated with gut dysbiosis and certain diseases. We also identified genetic variants of dozens of microbial taxa (e.g. a species, genus or family of bacteria). We studied nine cardiometabolic traits (weight, blood pressure, blood lipids, insulin, etc.) as well as eight chronic diseases: Alzheimer's disease, depression, Type 2 diabetes, fatty liver disease, atherosclerotic coronary artery disease, stroke, osteoporosis and renal failure. We also studied the effect of these factors associated with gut microbiota on healthy life expectancy and longevity.
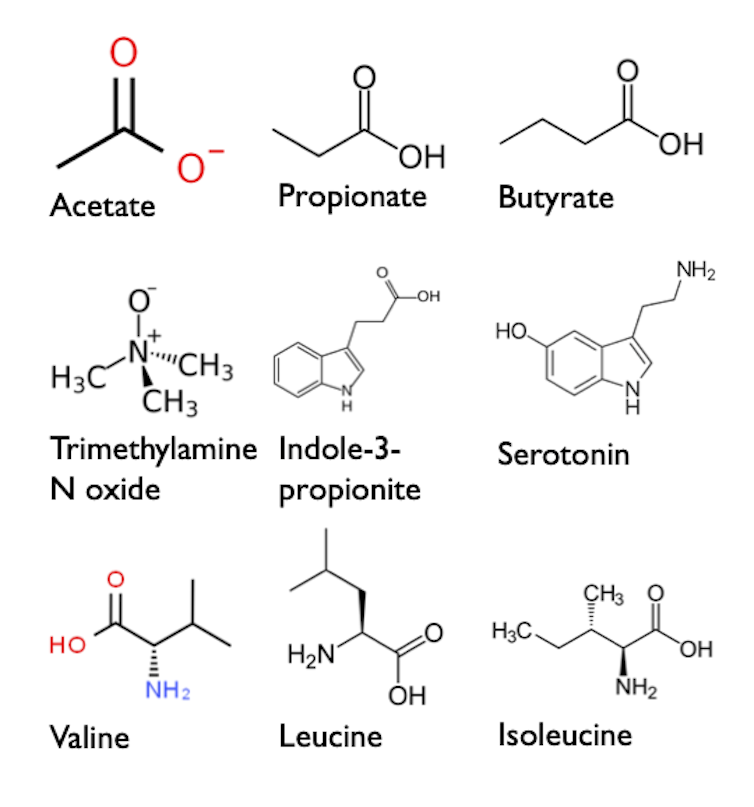
Small organic molecules called metabolites are produced by gut bacteria. These molecules could reach organs such as the liver and the brain. However, their role in the development of chronic diseases and life expectancy is controversial. (Benoît Arsenault), Fourni par l'auteur
We hypothesized that, in the light of previously published data, a causal link would be revealed between gut dysbiosis and chronic diseases associated with aging.
However, contrary to our hypothesis, this Mendelian randomization analysis did not show significant effects of gut microbiota on metabolic factors and chronic diseases. Seven associations between certain microbial parameters and chronic diseases associated with aging appear to be potentially causal, but their effect is small and we cannot rule out the possibility that these associations happened by chance. Overall, the results offer little support for the hypothesis that the gut microbiota has a significant effect on our weight, metabolism and risk of developing chronic diseases.
Results that call for caution
These results suggest that the previously observed associations may not be causal. The associations could be explained by the diseases themselves (reverse causality bias) or by confounding factors (confounding bias) such as diet, medication, smoking, metabolic health or others. However, these findings are consistent with the results of four recent randomized clinical trials showing that transferring gut microbiota from thin to heavyweight individuals does not lead to any weight loss or significant improvement in metabolic profile.
Mendelian randomization is a method that has several advantages over observational studies. However, these results need to be contextualized. It is entirely possible that the genetic parameters we used to predict the metabolites and microbial species associated with gut dysbiosis do not fully capture the complexity of the gut microbiota. This would diminish our ability to identify meaningful associations. Therefore, studies with larger sample sizes and better characterization of the gut microbiota and its metabolites will be needed to determine whether certain gut bacteria play a key role in the etiology (the study of causes) of chronic disease and longevity.
Although the impact of gut dysbiosis on chronic disease appears to be limited, gut health is important for other aspects of human health. For example, the microbiota prevents other harmful bacteria from colonizing our gut. In addition, it allows us to digest certain nutrients (e.g. dietary fiber) that would otherwise be rejected by our bodies.
Therapies that modulate the gut microbiota have recently been approved by U.S. health authorities for the prevention of C. difficile infections (a bacterium that causes diarrhea and other serious intestinal diseases). Our results, along with results from clinical studies less prone to reverse causality and confounding bias, do not, however, support a significant effect of gut dysbiosis on chronic disease.
These results support the conclusion that the potential of the microbiota as a therapeutic target for chronic diseases is, at present, low. We urge health professionals and the general public to be cautious about diagnostic tests based on gut microbiota to diagnose health problems that are not validated by the relevant health authorities.
Most importantly, we urge health professionals to avoid recommending specific interventions based on the mere fact that they would influence the parameters of the gut microbiota.
Éloi Gagnon, PhD Candidate, Université Laval and Benoit Arsenault, Chercheur au Centre de recherche de l'Institut universitaire de cardiologie et de pneumologie de Québec et Professeur titulaire au Département de médecine, Université Laval
This article is republished from The Conversation under a Creative Commons license. Read the original article.


Shares