It’s a little mind-boggling to think about, but there was a time when no stars existed in the universe. The earliest stars, galaxies and black holes came into being in a wondrous period called “cosmic dawn,” some 250 to 350 million years after the Big Bang.
All sorts of ingredients of our universe were popping into existence at that time: stars, galaxies, black holes. Given all the components were there, could that short list include life itself? Could aliens have popped up much earlier in the universe’s 13.8 billion year history?
The question of how life first came into existence has exercised scientists and philosophers for millenia. In a 2016 book on the subject, Sean Carroll describes how Jan Baptist van Helmont, a 17th Century chemist, thought that “the way to create mice from nonliving materials is to place a soiled shirt inside an open vessel, along with some grains of wheat.” After about twenty-one days, the wheat would supposedly have turned into mice.
“If for some reason you wanted to make scorpions rather than mice, he recommended scratching a hole in a brick, filling the hole with basil, covering with another brick, and leaving them out in sunlight.”
As Carroll goes on to say, “if only it were that easy.” One interesting angle on the question might be to go back not to the early years of Earth, but further — to those earliest millions of years after the Big Bang, when gravity essentially turned on the lights, pulling “us” out of the dark ages of a hot, dense and boring early universe into a cooler, more complex reality.
"One hundred million years after the Big Bang, there were pockets of enriched material that could have led to planets and life as we know it, potentially,"
Avi Loeb, director of the Institute for Theory and Computation at the Center for Astrophysics co-operated by Harvard University and the Smithsonian, and a theoretical physicist focused on cosmology and astronomy, told Salon that with some creative thinking, it might be possible to find evidence that life started far, far earlier than the earliest evidence we have for it on Earth.
“I would say one hundred million years after the Big Bang, there were pockets of enriched material that could have led to planets and life as we know it, potentially,” Loeb said.
Want more health and science stories in your inbox? Subscribe to Salon's weekly newsletter Lab Notes.
After all, that’s when the essential elements that make up life first appeared in our universe. Rooting around just in our solar system, we’re already finding evidence of the building blocks of life in unexpected places. In December, scientists studying findings from the Cassini mission (which sent a space probe to Saturn and its system in 1997, wrapping up in 2017) uncovered evidence of hydrogen cyanide on Saturn’s moon Enceladus. So if we’ve already found water, carbon dioxide, methane, ammonia and hydrogen gas on Saturn’s icy moon — which scientists predict are some of the crucial elements necessary for life to spring into being — would it be possible for them to create life much earlier in our universe’s evolution?
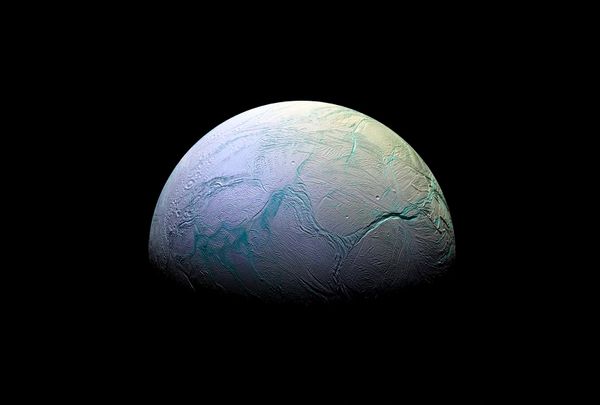 Saturn's moon Enceladus (Universal History Archive/Universal Images Group via Getty Images)
Saturn's moon Enceladus (Universal History Archive/Universal Images Group via Getty Images)
The life-giving elements emerged gradually after the Big Bang, about 380,000 years after the explosion, when the universe cooled enough for hydrogen atoms to form. For the next fifty to a hundred million years, space was completely dark, with hydrogen atoms spread across the universe, a gas that was eventually cleared – or ionized – by the ultraviolet light of the first generation of stars.
And then came the epoch of reionization, which lasted until about 100 billion years after the Big Bang, with new elements like carbon, oxygen, nitrogen and iron released from those first, massive stars,.They quickly exploded, giving way to a second generation formed around those heavy elements and others like cobalt and nickel, sulfur and silicon. Neutron stars merge to produce gold and uranium. The universe is full of stuff.
The region of habitability
But that’s not all you need to spark life. What about an atmosphere? Can’t forget the thing that lets us breathe and stay unbaked from solar radiation. For liquid water to exist — so as to have the chemistry necessary for life in a form we might recognize — you need external pressure. It can’t be done in a vacuum. Given the necessary pressure, you need a certain temperature. So the whole concept of a “habitable zone” for life is a Goldilocks one: in Loeb’s words, “Just the right distance [from a star], not too close so that it’s too hot, and not too far from the furnace so the surface freezes.”
Earth, however, has always – human-caused global heating aside – had a tendency to freeze, being a little outside the optimal position with respect to our own sun.
The clever Youtube channel Kurzgesagt produced speculation about whether the life that exists on Earth might not in fact have originated way, way back and far, far away, some time during the cosmic dawn and in some other part of the universe, draws in part on Loeb’s theorizing about the early post-Bang universe. Basically, considering the requirements for a habitable zone conducive to life, Loeb realized that you can get around the requirement of being close enough to a star to be optimally warmed. You’ve just gotta go back in time. Because back then, the universe was not just smaller. It was hotter, too.
“In the early universe, that temperature requirement could have been met when the universe was just fifteen million years old,” Loeb said. “And that would allow liquid water to exist, or [an adequate temperature could be achieved] when it was about seventy-five million years old or so, when liquid methane or ethane would have existed just like in Titan.”
“It’s just the temperature of the entire universe because it’s filled with the radiation background, or the cosmic microwave background [...] so you don’t need the object to be close to a star to attain this temperature. It would have been everywhere.”
Life as we don’t know it
When you think about the building blocks of life, typically you need water. But there are potentially other solvents that could do the job: methane or ethane, for example.
After all, why assume that, if there’s life out there in the vast, unknown reaches of the universe, it follows the same contours as ours? Sure, the laws of physics place certain constraints on the pre-conditions for life – but there’s no reason to be so anthropomorphic, or terramorphic, about it.
Loeb cited the Dragonfly mission, currently scheduled by NASA to launch in 2028 to explore Saturn’s largest moon, Titan (as well as Enceladus, which is Saturn’s sixth-largest moon, another candidate for life in our solar system is Jupiter’s moon, Europa).
Loeb describes the Dragonfly mission as a fishing expedition. Literally: looking for alien fish.
“You go there and you look for fish, and if there is something moving and alive, that would be amazing,” Loeb said. “Because not only would we realize that life exists elsewhere, but also that it could take very different forms. Of course, I would not recommend putting these fish in restaurants on Earth and eating them, because it might not be good for our stomachs. But you can imagine — I mean, we just don’t understand how life emerged on Earth with its complexity and definitely not in other liquids.”
If we were to come across life based on solvents other than water, Loeb explained that “would open up a whole new frontier of biology to understand what happens in methane and ethane. And maybe it will lead to some important insights about life.”
It’s actually not just the temperature but a temperature gradient that seems to be needed to kickstart the initial reactions needed, but Loeb argues that under the surface of an object like a planet, you can get higher temperatures where liquids might hide, as we believe they do on Europa and Enceladus, for example, which are frozen on the surface due to their great distance from the sun, but which conceal liquid oceans buried under the ice.
“I mean,” said Loeb, “your life is as boring as you are, if you don’t have imagination.”
To be fair, though, Loeb has been accused of having a little too much imagination before, in particular when he was quick to suggest that ‘Oumuamua’, a mysterious, highly reflective space rock (or chunk of space ice) briefly pulled by the sun’s gravity into our inner solar system in 2017, might in fact be an artifact from an extraterrestrial civilization. But while Dr. Catherine Neish, associate professor in Planetary Surfaces at the University of Western Ontario, and a co-investigator on the Dragonfly mission, might not be looking for fish on Titan, her expedition will, she hopes, turn up at least the building blocks of life: amino acids. What she’s really interested in is prebiotic goo, the stuff from which life first arose – and which she can mimic with lab-created analogues (non-identical copies) of the kind of chemicals found in the haze in Titan’s organic chemical-rich atmosphere. During her PhD work she discovered that when you mix such chemicals with water, “you can make some really interesting products that are of a biological or prebiotic nature.”
“You take methane, nitrogen, you spark it with electricity, you make these haze analogues,” she told Salon in an interview, referring to Titan's thick, gassy atmosphere. “So no oxygen in there. It should be just carbon, nitrogen, hydrogen, those three elements. But then if you add them to water, they can react to form more interesting biological molecules. I was especially interested in amino acids and nucleotides which make up proteins and nucleic acids.”
While water is frozen most of the time on the surface of Titan, where it’s around -288.67º F on a balmy day, there are certain environments even there where you can heat up the rock enough that liquid water could exist – and thus the oxygen (part of the H2O molecule) that we need to have a hope of life. One of those environments would be the kind that exists after a comet strikes the moon’s surface, melting it, resulting in transient liquid water at the bottom of the impact crater it creates.
“You know, how far could you get towards life?” That’s the question Neish asked with the highly interdisciplinary research she conducted (working with chemists) for her PhD in Planetary Sciences, concluding that it wasn’t all that difficult to make prebiotic molecules like amino acids in such environments. Back when she graduated in 2008, actually going back to Titan to look for life there — whether Loeb’s hypothetical alien fish or a nice string of amino acids — seemed about as unrealistic as going back in time in search of the perfect cosmic microwave background to incubate life.
We need your help to stay independent
But then in 2016, Neish got word that Titan had been added to the NASA New Frontiers Program. And, a proposal and a long selection process later, Neish and her team are working on a plan to look for evidence of prebiotic chemistry in the wild, on an impact crater on Saturn’s moon.
“In the lab we have these experiments running for days, weeks, months at the most. Whereas Titan, these experiments have been happening for billions and billions of years. So you know, just how advanced can you get with prebiotic chemistry in a natural environment?” Neish asked.
It’s not just a hope of finding life on Titan that either scientist is thinking of, but the hope such a find on Titan might represent complex and interesting life one day being discovered elsewhere in the universe. And not just after the Big Bang, but in a galaxy far, far away.
“There’s so many mysteries about the environment in which life arose on Earth,” said Neish. “Because that Earth doesn’t exist anymore. So we can go to other planets like Titan and maybe it’s more representative of what the chemistry was on the early Earth, a billion years ago. And so by learning about what steps do we need to take to originate life, it tells us more about how life came to be here on Earth, but also elsewhere in the universe, on other planets.”



Shares